Basic Science
1. Ovarian Development and Primary Ovarian Insufficiency
Germ cells carry the history of our generation(s), are unique in their biology and are of utmost importance in propagation and causation of genetic disorders. Their uniqueness is also reflected in special mechanisms and molecules that govern their differentiation and growth. Our lab is particularly interested in understanding the transcriptional regulation of ovarian follicle activation and oocyte survival.
Early stages of ovarian follicle formation, beginning with the breakdown of germ cell cysts, the formation of primordial follicles, and transition to primary and secondary follicles, are critical in determining the reproductive life span and fertility. Transcription of numerous germ cell-specific genes necessary and essential for follicular development is initiated during these early stages of follicle formation. These transcription factors are necessary to drive oocyte growth and synthesis of maternal effect genes that determine early embryogenesis and are likely involved in the setting of epigenetic marks. We discovered novel germ cell-specific transcriptional regulators Sohlh1, Sohlh2, Lhx8, and Nobox. We also discovered that mutations in oocyte-specific transcriptional regulators such as Nobox and Figla associate with premature ovarian failure, emphasizing the importance of these pathways to women’s health. Our lab is additionally interested in studying MCM8 and MCM9.
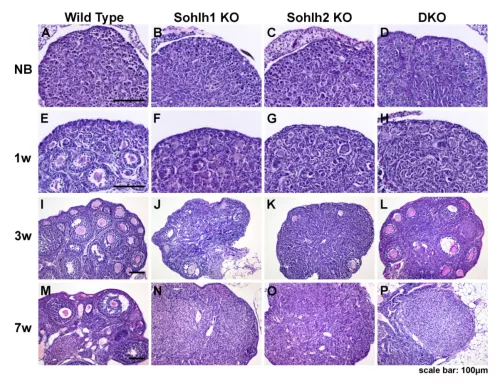
Sohlh1 and Sohlh2 are basic helix-loop-helix transcriptional regulators that suppresses primordial follicle activation (PFA), and Sohlh1 and Sohlh2 deletions cause rapid PFA and oocyte loss. LHX8, a highly conserved LIM homeodomain protein, is located downstream of SOHLH1 and SOHLH2, and also represses primordial follicle activation. SOHLH1, SOHLH2 and LHX8 are uniquely expressed in the germline, and their deficiency affects gonadal development. We are exploring the various effects of these genes within mouse models in our laboratory.
MCM8 and MCM9 are members of the minichromosome maintenance (MCM). These are autosomal genes that have been implicated in causing hypergonadotropic hypogonadism in an autosomal recessive pattern. Female mice with defects in MCM8 and MCM9 are infertile, with early loss of germ cells and a high predisposition to hepatocellular carcinoma and ovarian tumors. It is also suggested that this gene may strongly correlate with menopause. We have developed mouse models in our lab to study the fertility of these mice and understand their effect on female infertility and aging.
In addition to uncovering mechanisms of oogenesis, we are interested in exploiting tissue-specific pathways to modulate fertility and reproductive life span and measure ovarian reserves. We are specifically interested to identify novel biomarkers of primordial follicle reserves as well as tissue-specific targets to control reproductive life span.
2. Uterine leiomyomas
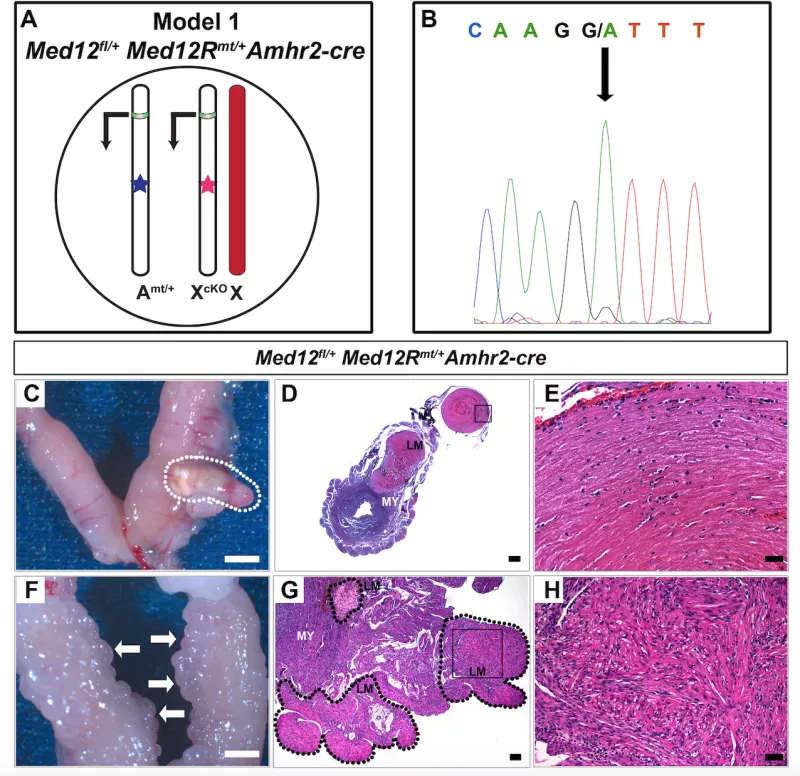
Uterine leiomyomas, better known as fibroid tumors, are clinically apparent in nearly 25% of women by age 45, and they cause major morbidity in American women. More than 200,000 surgeries are performed each year to either remove the leiomyomatous tumors (myomectomy) or the entire uterus (hysterectomy). Approximately half of all leiomyomas are asymptomatic, while the rest cause pelvic pressure and pain, irregular and heavy bleeding, anemia, premature labor, and infertility. These symptoms are intensified by the common occurrence of multiple tumors within a single uterus, often necessitating surgical intervention. We have discovered microdeletions and duplications that associate with this tumor as well as that mutations within one gene, Med12, are present in as many as 70% of women with leiomyomas. We are currently investigating the mechanisms of Med12 action in leiomyomas and therapies directed towards eliminating such tumors in symptomatic women.
Translational Science
1. Genetics of Human Gonadal Insufficiency
Idiopathic POI affects 1-4% of women, and is clinically defined as a cessation of menses prior to age 40 (normal being 50-52), with elevated follicle-stimulating hormone (FSH) levels and low serum estradiol levels. POI is rarely part of a genetic syndrome and more commonly is idiopathic and non-syndromic. Women with POI present with amenorrhea, either primary or secondary, hot flashes, and vaginal dryness. A long term consequence of POI is 50% higher overall mortality as compared to women who reach natural menopause, with an 80% increase in mortality due to ischemic heart disease and an increased risk of cognitive impairment and dementia, as well as premature osteoporosis. There are major gaps in our knowledge regarding genetics, natural history, and medical management of primary ovarian insufficiency. Reliable biomarkers that can predict reproductive life span and ovarian failure are needed. Cryopreservation techniques of ovarian tissue and oocytes have advanced tremendously, and offer fertility preservation options to women at risk for premature loss of ovarian function. There is a great need to identify women who are at risk for ovarian failure, and who can benefit from advanced fertility preservation technologies. Our laboratory is interested in determining genetic markers of ovarian aging. We are recruiting women who were diagnosed with ovarian insufficiency/failure prior to age 30, and whose cause of ovarian failure is unknown. If interested in participating in this study, please email [email protected] for further information.